PHARMACEUTICAL ZONE DEVELOPMENT
ARABOKKA-HAMBANTOTA
Hambantota
LOCATION
100 Acres
Land Extent
Project Overview
The BOI, in order to support developing new industry verticals via import substitution and exports, is establishing a state-of-the-art pharmaceutical zone in Arabokka, Hambantota. This dedicated Pharmaceutical Manufacturing Zone plans to meet 40% of the domestic demand for pharmaceutical products by locally manufactured drugs while creating a pathway to export earnings as import substitution by 2025.This State–of–Art zone will be designed to International standards and accreditations. This specifically tailored zone will make a conducive and liveable business environment to globally renewed pharmaceutical brands to bring the latest technological advances to manufacture pharmaceutical products.
- Pre-approved zone: All environmental clearances to manufacture a full suite of pharmaceutical products and fast tracked NMRA approvals.
- Strategic location: Proximity to both the Hambantota port and Mattala airport, with dedicated facilities at airport to facilitate sea-air logistics.
- Common facilities provided: Wastewater treatment & sea outfall and common logistics facilities
Project/ownership structure
Foreign Direct Investments (FDI), Domestic Private Investment

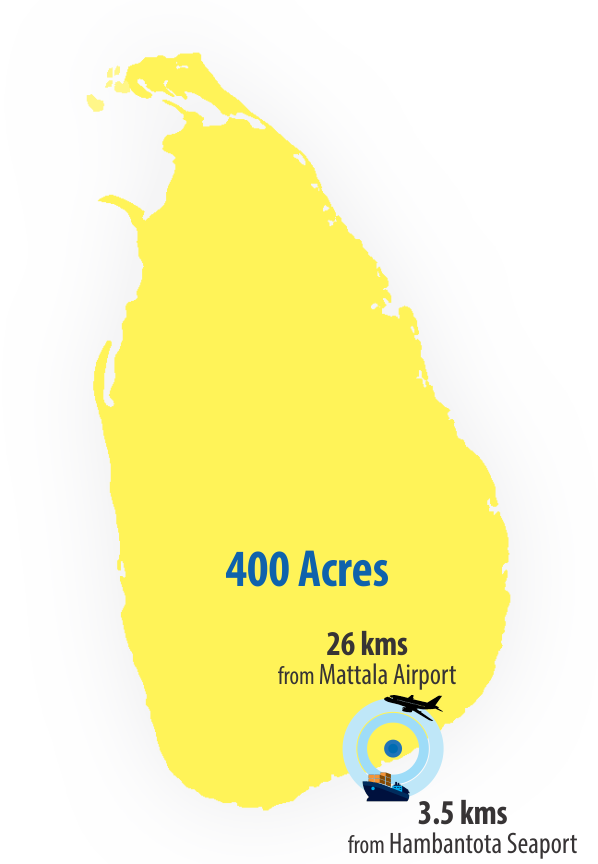
Strategic location
Proximity to both the Hambantota port and Mattala airport, with dedicated facilities at airport to facilitate sea-air logistics.

Suggested Activities
Software Development
Research & Development
Technology Incubators
BPO, KPO
